Search
Günter Hager 1943-2017
DOI: 10.17160/josha.4.1.272
On 19 February 2017, Prof. Dr. Günter Hager, member of the JOSHA Editorial Board for the Section "Philosophy, Ethics and Law", died at the age of 73 years. He contributed decisively to the success of JOSHA in the conception of our journal, the content design, and development, as well as his own important contributions. Until his retirement in 2011, he was a professor of civil law with a focus on International Private Law. After his retirement, Günter Hager increasingly dealt with questions of ethics and the environment and nature conservation, publishing many of his great works in JOSHA. With him, we lost not only a great legal scholar and an important editor of JOSHA but also a wonderful friend who was broadly interested in literature and music and whose narratives were, as it were, creative, humorous, and linguistically refined. One of his favorite aphorisms from his inexhaustible fund of quotations was "What is to be dismissed this minute, no eternity will return.
Enough. Concerning me. - Summer School 2017 in Wittenberg
DOI: 10.17160/josha.4.1.271
Enough. Concerning me. Different facets of one statement – encouraging and setting limits at the same ime. We want to arouse curiosity, stimulate new approaches and think out the future. At the Summer School 2017 in Wittenberg, in the middle of the Reformation anniversary, we want to broaden our horizons and get personal: letting ourselves be concerned. On over 100 pages we have collected details on the seminars, workshops and events for you. They focus on the general topic – and are interdisciplinary, outward-looking and interreligious. You can see it from the structure of this booklet: just as the German and English appear side by side, in the Reformation Summer many educational experts will complement and challenge one another with international perspectives in over 30 seminars. Their individual styles are represented in the seminar descriptions. The general topic arose from some hard thinking by undergraduates and doctoral students exactly a year ago.
Ethnic Identity from a Macro-Sociological Perspective: Second-generation Iranian “immigrants”
DOI: 10.17160/josha.4.1.266
A feeling of chosenness can result in isolation from other nations and ethnic groups. As it seems, such a feeling of ethnic chosenness may have led to a tendency toward ethnocentrism and in some extreme cases, towards racism found among some Iranians (30%-40% according to the world value survey). Such an ethnocentrism may have negative effects on the identity construction of young people, particularly those outside Iran. In the present article, based on studies conducted among Iranian-Swedish and Iranian-American young people, I will try to shed light on this problem. Key words: Racism, Iranians, Second-generation “immigrants”, Ethnocentrism, National identity, Ethnic identity
Short Stories Series: "Prom Night"
DOI: 10.17160/josha.4.1.265
This article is the first of a series of short stories by the young author Zazie-Charlotte Pfeiffer, who received the "Jean-Paul" Award in 2013 and the "Tom Sawyer" Award in 2012 for her work. SUMMARY: Marco has always been a dreamer and the creator of his own little world. Tonight he is dreaming and reflecting about past and future, lets his gazes fly over a sleeping city and searches for roots that he didn’t even know he lost. A glimpse of future possibilities, a moment of breath in silence and the reflections of a stranger on his own life. INSTITUTION: Albert-Ludwigs-University Freiburg im Breisgau, Germany.
Complexity of Iterative Model - Architectural Integrated Design as an Evolutive Transdisciplinary Strategy. Case Study: A City Without a River
DOI: 10.17160/josha.4.1.264
Contemporary challenges should encourage new explorations in order to administer new urban solutions; thus, assuring better and higher quality of life. The meaning of transdisciplinary design consists of different professions closely related to the architectural design targeting for better and qualitative design solutions, which with new findings exceed the conventional and traditional disciplinary barriers. This study investigated the downtown of Prishtina city focussing on urban design issues, pollution, ecology, and sustainable urban design. The research method consists of empirical observation through wide centre zone with an accent to the urban water management plan as a contemporary reflection to the past decisions of Prishtina municipality.
Architectural Reflection on Italo Calvino’s Invisible Cities
DOI: 10.17160/josha.4.1.261
Cities actually endure a considerable lack of space for much needed urban development. Moreover, nowadays contemporary architectural challenges are extensive and complex social issues. Furthermore, present challenges represent fundamental city necessities or a broad of society demands, which we must take into consideration. Hence, we must make the necessary preparations for direct urban actions on fulfilling those issues from the best possible known urban strategies. Urban planning is a design process with a primary course to protect the environment, to manage urban infrastructure as a whole integrated system, and deliver the most appropriate style for living. In relation to sustainability and ecology, a qualitative urban design can significantly improve condition, and quality of life of urbanites. This study, has conceptually researched a design model of high-rise structures with its proportions mainly focussing on future urban planning structures.
Ion Channels: Their Discovery and their Role in Pharmacology and Biomedicine
DOI: 10.17160/josha.4.1.260
The concept of bioelectricity emerged in the late 18th century, based on the experiments of Galvani and Volta. Sixty years ago Hodgkin and Huxley showed that the nerve impulse is a result of permeability changes of the nerve membrane. This provoked the question of what the molecular mechanisms of such permeability changes might be. In 1976, Bert Sakmann and myself were able to show that the so-called ion channels –proteins that gate ion fluxes across membranes- mediate these responses. Research over the last 40 years has shown that ion channels are not only present in electrically excitable cells, such as in nerves and muscles, but also in basically all cell types of our body, mediating a variety of physiological functions. We now know that they are prime drug targets and that dysfunction of ion channels underlies a variety of diseases.
JOSHA Table of Contents - Volume 3 Issue 7
DOI: 10.17160/josha.3.7.259
We are closing the last issue of 2016 with five novel contributions to the scientific, humanities, and arts fields. We expect that you will enjoy this last issue of the year, and we are already looking forward to giving 2017 the opportunity to surprise us with new content, authors, readers, and memberships. Since the foundation of our journal in December 2014, our articles have reached almost 120,000 views and over 95,000 downloads, and the most viewed article has reached 2,800 views and 1,800 downloads showing the reach of JOSHA published articles and the continuous growth of the JOSHA community.
Challenges of Architectural Design in relation to Environment and Air Pollution. A Case study: Prishtina’s first public parking garage
DOI: 10.17160/josha.3.7.254
Cities are complex ecosystems with specific phenomenon directly reflected in our health, resources, economic, social and aesthetic fields. It can be conceptually considered that cities are locally and regionally specific. Urban planning is a process with a primary role to protect and use of environment, to manage spatial planning and urban infrastructure as a whole system. In relation to sustainability and implementation of multi-level law reinforcement, urban planning and design can significantly improve quality of life of their urbanites, particularly in relation to air pollution. Surely, long-term plans and strategies have been adopted in Kosovo, but the challenges will remain in implementation and in enforcement of these administrative instructions. Therefore, it is crucial to encourage every action, related to city functionality which will minimize air pollution.
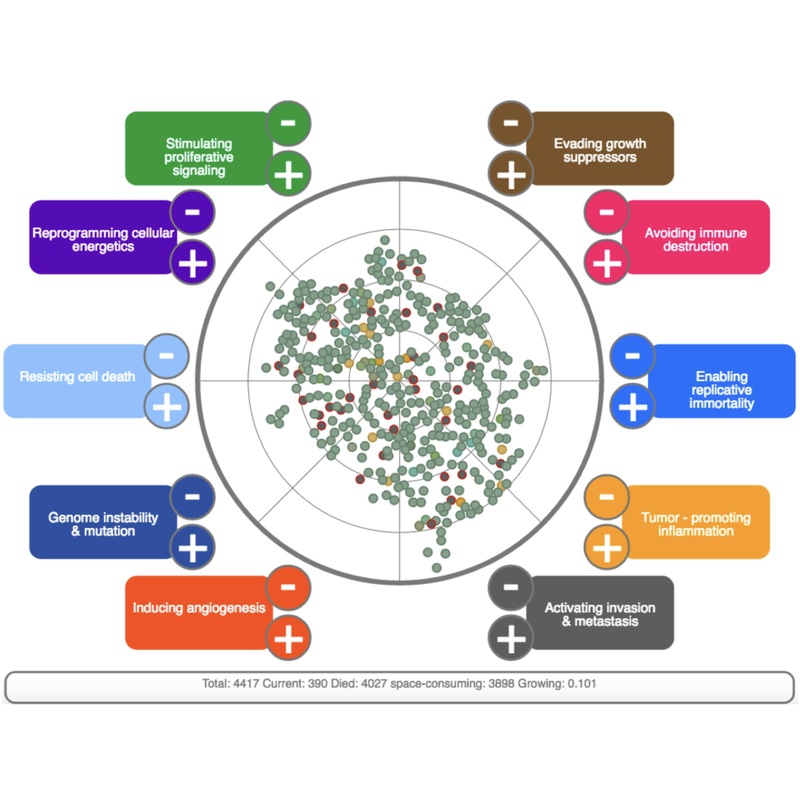
Towards Simulating Carcinogenesis: Modeling and Simulating Carcinogenesis, Hematopoietic Tissue Homeostasis and Leukemogenesis
DOI: 10.17160/josha.3.7.253
The previously identified cancer hallmarks (Groten et al. 2016, DOI: 10.17160/josha.3.7.252 ) were described by mathematical algorithms. Subsequently, a computational simulation of carcinogenesis has been developed. In the next step, the proposed algorithms and correlations have been tested, validated and adapted through the simulation (http://mertelsmann.psiori.com/). In a second model, we transferred the insights won from the first simulation to the simulation of hematopoiesis tissue homeostasis and leukemogenesis (http://hem-model.psiori.com/hema_simulation). Our findings indicate that the 10 “Hallmarks” proposed by Hanahan and Weinberg can be assigned to two major groups, “Growth/Apoptosis Balance” and “Genetic Fidelity, Immortality”. Modeling Hematopoiesis revealed one missing Hallmark, “Block of Differentiation”, which we propose to assign to the broader term “Stem Cell Features”.






.jpg?1485870632)
.jpg?1478721409)